Medical devices for mechano-therapy & regenerative medicine
Mechanical stimulation (for example, massage) is recognized for its role in modulating various biological processes at both cellular and tissue levels. However, its potential in medical applications for tissue regeneration and rehabilitation has been constrained by the lack of appropriate devices. We aim to address this by developing medical devices that interface directly with tissue. These devices, embedded with soft actuators and sensors, will deliver targeted mechanical stimulation to injured tissue, paving the way for novel therapeutic interventions.
Relevant publications
Nam, et al., Active tissue adhesive activates mechanosensors and prevents muscle atrophy, Nature Materials (2023)(Link)
S. Nam and D.J. Mooney, Polymeric tissue adhesives, Chemical Reviews (2021) (Link)
Seo, et al., Skeletal muscle regeneration with robotic actuation-mediated clearance of neutrophils, Science Translational Medicine (2021) (Link)
Freedman, et al., Enhanced tendon healing by a tough hydrogel with an adhesive side and high drug-loading capacity Nature Biomedical Engineering (2022) (Link)
Implantable medical devices for mechanical stretching/contraction stimulation



We developed a novel class of medical devices designed to deliver mechanical stimulation within the body. Our findings indicate that stretching/contraction stimulation can either prevent or significantly delay muscle atrophy. The accompanying video showcases the implantable device in action, administering mechanical stretching and contraction stimulation to mouse muscle.
Robotic actuation systems for compressive stimulation
We discovered that compressive stimulation, like massage, can enhance the healing process of injured muscles by modulating the immune response. This underscores the therapeutic efficacy of mechano-medicine.

Tissue viscoelasticity

We found a unique time-dependent mechanical behavior in tissues: the greater the strain, the faster the relaxation — a phenomenon we termed ‘strain-enhanced stress relaxation’.
Molecular mechanisms underlying tissue viscoelasticity

We identified potential mechanisms underlying tissue viscoelasticity and leveraged our insights to develop better cell culture platforms.
Tissue & soft materials mechanics
Biological tissues and materials exhibit distinct mechanical behaviors, including nonlinear elasticity, viscoelasticity, plasticity, and toughness. While these properties are evident, the precise molecular mechanisms driving them remain elusive. Our objective is to not only characterize these unique mechanical behaviors but also to shed light on the molecular processes underpinning them.
Relevant publications
Nam, et al., Cell cycle progression of cancer cells in confining hydrogels is regulated by a growth-responsive signaling axis, Science Advances (2019) (Link)
Nam, et al., Varying PEG density to control stress relaxation in alginate-PEG hydrogels, Biomaterials (2019) (Link)
Nam, et al., Strain-enhanced stress relaxation regulates nonlinear elasticity in collagen gels, Proceedings of the National Academy of Sciences (2016) (Link)
Nam, et al., Viscoplasticity enables mechanical remodeling by cells,
Biophysical Journal (2016) (Link)
Extracellular matrix mechanics and its impact on cells
The mechanical properties of the extracellular matrix, which occupies the space outside the cell membrane, regulate a wide range of cellular behaviors, including spreading, differentiation, matrix formation and remodeling, migration, and division. We study the mechanisms through which matrix mechanics impact cellular functions. With this knowledge, we aim to pave the way for innovative therapies and applications in the realm of regenerative medicine.
Relevant publications
Nam, et al., Cellular pushing forces during mitosis drive mitotic elongation in collagen gels, Advanced Science (2021) (Link)
Nam, et al., Cell cycle progression of cancer cells in confining hydrogels is regulated by a growth-responsive signaling axis, Science Advances (2019) (Link)
S. Nam and O. Chaudhuri, Mitotic cells generate protrusive extracellular forces to divide in three-dimensional microenvironments, Nature Physics (2018) (Link)
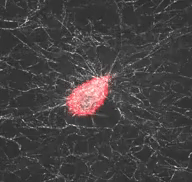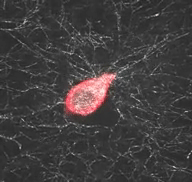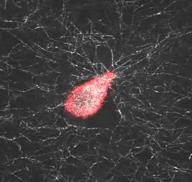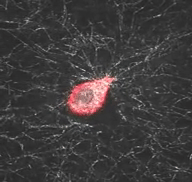
Mechanical interactions of cells with their microenvironment
The video demonstrates the dynamic mechanical interaction between a fibroblast (red) and the surrounding network of collagen fibers (white)
Proposed mechanisms for tumor growth in viscoelastic matrices

We discovered novel molecular pathways that drive tumor growth in viscoelastic matrices, shedding light on the pivotal roles of mechanics and force generation in the process. These insights pave the way for a platform aimed at discovering therapeutic interventions for a wide range of diseases, including cancer.
